In contrast to the one-size-fits-all medicine, personalized medicine aims to tailor treatment to the individual characteristics of each patient. This requires the ability to classify patients into subgroups with predictable response to a specific treatment. The field of pharmacogenetics /pharmacogenomics has made important contributions to this problem for more than 50 years. Ideally, personalized medicine will enable targeted prescription of any given treatment to only the likely responders, to avoid adverse reactions and expensive treatments in non-responders. Although there are already many examples of personalized medicine by leveraging genetics/genomics information in current practice, such information is not yet widely available in everyday clinical practice, and is insufficient since it only addresses one of many factors affecting response to medication.
With the tremendous growth of the adoption of EHR, various sources of clinical information (e.g., demographics, diagnostic history, medications, laboratory test results, vital signs) are becoming available about patients. Recently, some treatment comparison studies were conducted based on data from EHR of a cohort of clinically similar patients who received the treatments previously and whose outcomes were recorded. There are also some studies, of combining clinical and genetics/genomics information in selecting optimal clinical treatments. Existing approaches using clinical information for personalized medicine rely on large amounts of real-world data regarding the target treatment itself, which may not be available for new drugs or rarely-used treatments.
Drug similarity analytics aims to find drugs which display similar pharmacological characteristics to the drug of interest. The similarity analytics is usually conducted based on one or more types of drug characteristics (e.g., chemical structures, biological targets, indications, side-effects, and gene expression profiles). Drug similarity analytics has been widely used in drug repositioning, drug side-effects prediction, drug-target interactions prediction, and drug-drug interactions prediction, applications. This approach has been shown to deliver competitive or even better accuracy to more complexes, feature-vector-based methods, (e.g., support vector machines, random forests). In this study, we used drug similarity analytics to transmit EHR clinical information from well-studied drugs (i.e., drugs with many EHR records) to rarely-studied drugs (i.e., drugs with no or few EHR records).
Patient similarity analytics aims to find patients who display similar clinical characteristics to the patient of interest. The goal is to derive clinically meaningful distance metrics to measure the similarity between patients represented by their key clinical indicators. The resulting individualized insight of patient similarity analytics includes suggestions on how to manage care delivery to the patient (especially for patients has multiple diseases), and predictions of health issues that could arise in the future (because patients with similar characteristics had experienced such health issues). With the right patient similarity in place, patient similarity analytics have been used in the target patient retrieval, medical prognosis, risk stratification, and clinical pathway analysis tasks.
This analysis would involve constructing a heterogeneous graph which includes two domains (i.e., patients and drugs) and encodes three relationships (i.e., patient similarity, drug similarity and patient-drug prior associations), and propose a heterogeneous label propagation algorithm which can be used to generate personalized drug recommendations by leveraging patient similarity and drug similarity analytics. To our best knowledge, the heterogeneous graph formulation of the EHR data has not been proposed in any previous literature. The label propagation model over heterogeneous graph by leveraging both patient similarity and drug similarity analytics is also significantly different from existing label propagation models.
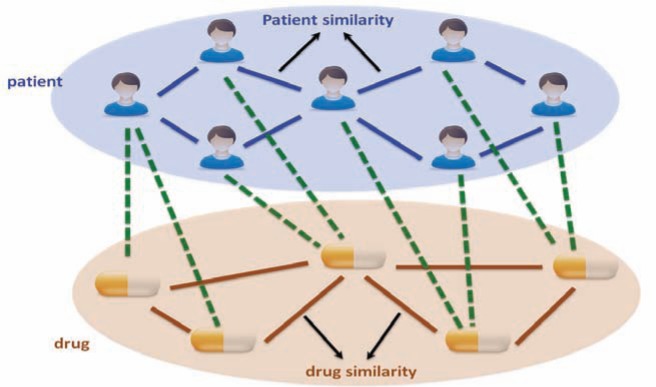
The rapid adoption of electronic health records (EHR) provides a comprehensive source for exploratory and predictive analytic to support clinical decision-making. In this paper, we investigate how to utilize EHR to tailor treatments to individual patients based on their likelihood to respond to a therapy. We construct a heterogeneous graph which includes two domains (patients and drugs) and encodes three relationships (patient similarity, drug similarity, and patient-drug prior associations). We describe a novel approach for performing a label propagation procedure to spread the label information representing the effectiveness of different drugs for different patients over this heterogeneous graph. The proposed method has been applied on a real-world EHR dataset to help identify personalized treatments for hypercholesterolemia. The experimental results demonstrate the effectiveness of the approach and suggest that the combination of appropriate patient similarity and drug similarity analytics could lead to actionable insights for personalized medicine. Particularly, by leveraging drug similarity in combination with patient similarity, our method could perform well even on new or rarely used drugs for which there are few records of known past performance.
Drug Personalization
So, the basic question we want to answer for personalized medicine is “whether drug A is likely to be effective for specific patient B”. To take into consideration the specific condition of patient B as well as the characteristics of drug A, we propose to leverage the information of the patients who are clinically similar to patient B as well as the drugs which are similar to drug A. Moreover, we also considered the prior associations between patients and drugs, which were measured by the TC between ICD9 diagnosis of patients and ICD9-format drug indications from MEDI database (MEDI is an ensemble medication indication resource, which was created based on multiple commonly used medication resources by leveraging natural language processing techniques). In this way, we constructed a heterogeneous graph illustrated in, which includes two domains (patients and drugs) and encodes three relationships (patient similarity, drug similarity and patient-drug prior associations). In the following we present a concrete heterogeneous label propagation algorithm to answer the question proposed at the beginning of this paragraph.
India’s largest platform for AI & Analytics leaders, professionals & aspirants
3AI is India’s largest platform for AI & Analytics leaders, professionals & aspirants and a confluence of leading and marquee AI & Analytics leaders, experts, influencers & practitioners on one platform.
3AI platform enables leaders to engage with students and working professionals with 1:1 mentorship for competency augmentation and career enhancement opportunities through guided learning, contextualized interventions, focused knowledge sessions & conclaves, internship & placement assistance in AI & Analytics sphere.
3AI works closely with several academic institutions, enterprises, learning academies, startups, industry consortia to accelerate the growth of AI & Analytics industry and provide comprehensive suite of engage, learn & scale engagements and interventions to our members. 3AI platform have 16000+ active members from students & working professionals community, 500+ AI & Analytics thought leaders & mentors and an active outreach & engagement with 430+ enterprises & 125+ academic institutions.

